
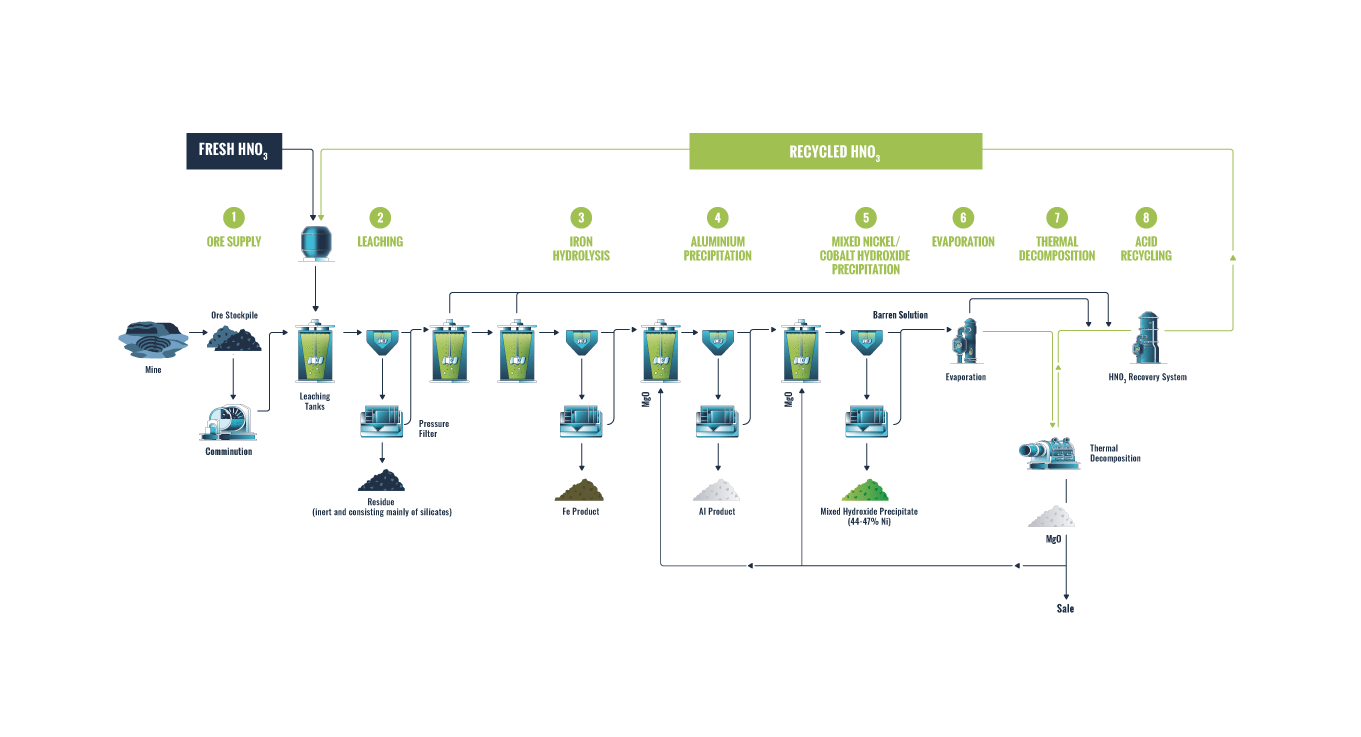
Blending: The DNi Process™ treats limonite, intermediate and saprolite ores in a single stream with no limit on the blend. This means that all of the laterite ore is used and allows for a lower nickel cut-off grade at the mine because more value is extracted, increasing the size and value of the resource.
Drying: The ore is dried to facilitate the comminution circuit and reduces the energy needed to evaporate off excess water.
Comminution: The ore is then passed through the comminution circuit where it is reduced to 100% passing 1mm.
0.9 to 2.5 tonnes of HNO3 (nitric acid) is added to each tonne of ore in the leaching circuit. The leach tanks operate at 110°C, just below the boiling point of the slurry. Most of this heat comes from the reaction of the acid with the ore. Leaching takes from 3 to 6 hours and results in a pregnant leach solution (PLS) – a heavily loaded acidic nitrate solution of the dissolved metals (including Ni, Co, Fe, Mg, Cr, Al and Sc) leaving around 10 – 15% solids. Leaching tanks are covered and operate at atmospheric pressure with all gases collected and sent to the acid recycling circuit.
The PLS is separated from the leach residue via Counter Current Decantation units (CCDs) followed by pressure filtration. The leach residue is made up of the insoluble minerals remaining from the ore such as silicates, aluminosilicates, and clays. MgO is added to the washed residue slurry neutralising the remaining free acid and increasing the leach residue to pH 7. This slurry is filtered, dry stacked and returned to the mine. The residue contains a small amount of magnesium nitrate (fertiliser) which assists revegetation of the site. The combination of solid/liquid separation, followed by pressure filtration maximises the nitrate to nitric acid recovery – a key feature of the DNi Process™.
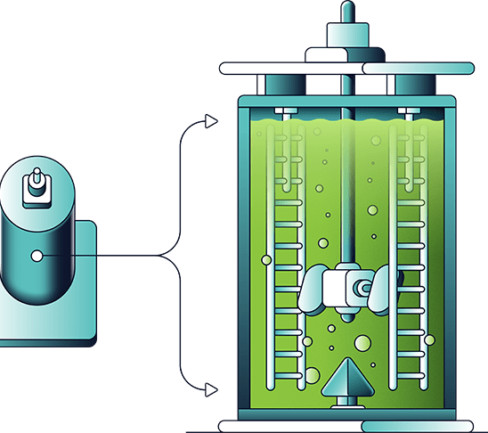
The leach solution is pumped to the iron hydrolysis circuit for iron removal. The PLS is heated near to its boiling point (120°C) where the vapour coming off the solution is water and nitric acid. As this water and nitric acid is removed the salt concentration increases which in turn increases the boiling point of the solution (boiling point elevation or BPE) from 120°C to about 165°C. The vapour that is distilled off is collected and recirculated to the acid recovery circuit. Once the solution reaches 140°C the ferric nitrate becomes unstable and decomposes to hematite (Fe2O3) – a saleable by-product of the DNi Process™.
Two stages of aluminium precipitation are used to minimise nickel and cobalt losses. MgO is added to the solution to raise the pH so that the aluminium begins to precipitate as a hydroxide. The second stage precipitation is returned to the iron hydrolysis circuit for redissolution targeting around 10-20ppm aluminium remaining in solution. The first stage product is thickened and filtered. The aluminium by-product contains most of the scandium present in the ore. It’s an intermediate product requiring further refining and can produce high purity alumina (HPA). It contains around 20-24% Al with small amounts of Fe, Mg, Ni, Co, Mn.
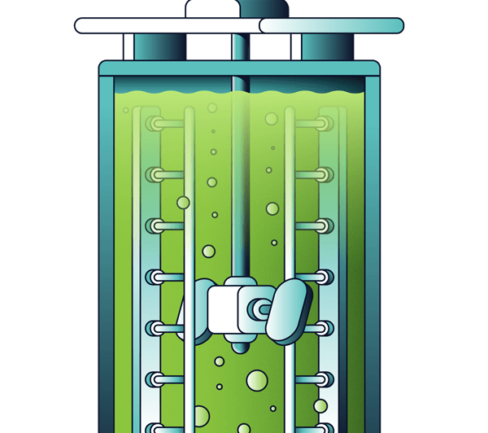
The PLS from aluminium removal moves to the MHP circuit where MgO is added to raise the pH and precipitate the nickel and cobalt. This is also done in 2 stages to remove of all potential impurities. The first stage MHP is thickened and filtered in the standard way. The second stage MHP is thickened and recycled to the iron hydrolysis circuit again for redissolution, ensuring that no nickel and cobalt remain. The remaining barren solution is the second stream from which the nitric acid is recycled.
The nickel content of the MHP is typically 40-45% and the cobalt content is around 1-3%. The MHP also contains some nitrogen (as nitrate). The MHP can then be refined at a different facility and converted into nickel and cobalt sulphates for use by EV battery manufacturers.
In the barren evaporation circuit, the barren solution is heated and water is evaporated. This water is recondensed in a heat exchanger and re-used within the plant as clean water. The barren solution undergoes a boiling point elevation (heated from 120°C in the first evaporator to 200-210°C). The resulting solution is magnesium nitrate-di to tri hydrate (Mg(NO3)2.2-3H2O), which is present as a hydrated molten salt and is easily pumped to the next circuit.
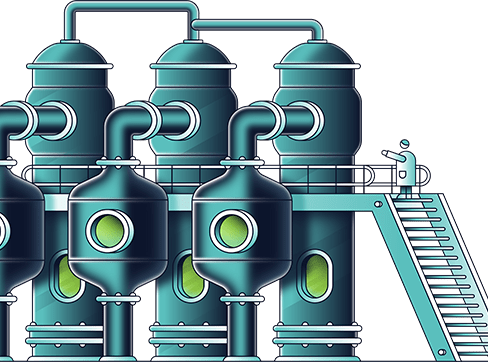
The concentrated barren solution is fed to the thermal decomposition unit where it is heated from a starting temperature of 200-210°C to over 500°C. It loses the remaining 2-3 moles of water and decomposes to MgO, NOx, and O2. The MgO is a solid product and the NOx and O2 leave the unit with the H2O as a hot gaseous stream which, together with other similar streams from other parts of the process, is converted to nitric acid in the next circuit. Another cost saving feature of the DNi Process™ is that some of the MgO produced in this circuit is used in the process plant as a neutralising agent, instead of buying lime or limestone. Depending on the ore blend there is typically significant excess MgO available for sale.
The hot gases from the thermal decomposition (O2, NOx and H2O) are cooled to re-condense the water and the stream is fed through the acid recovery columns where the NOx and O2 react with the water and form nitric acid. This patented process produces a high strength nitric acid – typically around 55-60% HNO3.
Over 99% of the initial acid used in the leaching process is recycled. The 5% makeup acid is typically 30-80kg of acid per tonne of ore. Because the majority of the acid used is generated by the recycling stage, the DNi Process™ is not limited to only treating low acid consuming ores.
90-98% of nickel and cobalt is extracted. Iron, magnesium, aluminium and scandium extractions depending on the mineralogy of the ore feed.